 |
|
|||||||||||
|
|
|
|||||||||||||||||||||||||||
The ChemSep ConsortiumThe ChemSep Consortium has been created to provide for the further development of the ChemSep nonequilibrium model. Benefits of membership include:




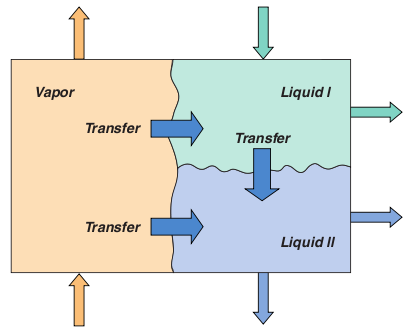
Three-Phase distillationThree-phase distillation remains relatively poorly understood compared to conventional distillation operations involving just a single liquid phase. Simulation methods currently in use for 3-phase systems employ the equilibrium stage model, although the accuracy of available thermodynamic models in predicting the highly non-ideal Vapor - Liquid - Liquid (VLL) equilibrium of these systems leaves something to be desired. It is important to be able to correctly predict the location of the stages where a second liquid phase can form (to determine the appropriate location for a sidestream decanter, for example). The limited experimental data available suggests that efficiencies are low and highly variable with between 25% and 50% being not uncommon. Clearly, a model based on the assumption of equilibrium on every stage cannot hope to be able to predict column performance. The material balances for a three phase system must allow for mass transfer to or from both of the other two phases. In addition, the model contains up to six sets of mass transfer rate equations. Three sets of equilibrium equations, one for each possible interface, must be included in the model. Estimating mass transfer coefficients and interfacial areas for three-phase systems is more difficult since there are no published correlations; hence theoretical approaches are necessary and are described in the references.
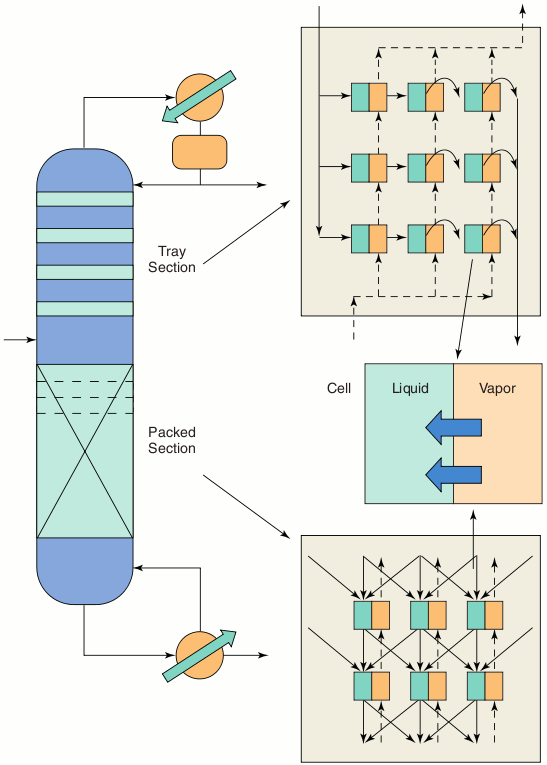
Improved Flow ModelsAn issue that is not adequately addressed by most models is that of vapour and liquid flow. Liquid flow patterns have a particularly strong influence on the performance of the column. ChemSep includes a dispersion model to better describe liquid flows in columns. Cell models also have been developed in which stages are divided into a number of contacting cells. Each cell describes a small section of the tray or packing, and by choosing an appropriate set of cell connections, one can very easily study the influence of flow patterns on the distillation process. A column of cells can model plug flow in the vapour phase, and multiple columns of cells can model plug flow in the liquid phase as depicted in the figure. Backmixing also may be taken into account. Flow patterns in packed columns (e.g. maldistribution) may be studied by means of a cell model. The objective of this phase of our work is to incorporate these models into Cape-Open ChemSep. Alternatively, ChemSep
Fundamentally sound mass transfer and hydrodynamic performance modelsThough ChemSep already comes with many mass transfer and hydrodynamic models, the need for more fundamentally sound models still remains. Most models were derived empirically via directly fitting tray efficiencies on geometry parameters and flowrates. As a result these models depend directly on the vapour flow or the tray weir height instead on those parameters that actually determine the mass transfer, such as bubble diameters which determine the bubble rise velocity and bubble interfacial area. Implicitely, the bubble diameter and velocity are functions of the vapour flow and weir height, but then with more interrelated dependencies on the tray geometry, physical properties, and flow rates than as used in the "1st generation" type of mass transfer correlations. Consequently these correlations are limited in their applicability and a large number of them is needed to model all the different kinds of separations. Furthermore, new enhanced capacity mass transfer internals now on the market operate differently and require new performance models. The ChemSep consortium develops more fundamental "2nd generation" models from first principles and tries to fit the parameters on available distillation and absorption test data to obtain a generic kind of model. Such new models are implemented in the ChemSep Model Developer which allows the user to add any new kind of internal or model. The layout parameters of new internals can consist of switches, multiple choice selections, or internals dimensions. The GUI can handle the entry for all. The program code for the new model or internal is compiled with the free OpenWatcom compilers and then is linked dynamically to ChemSep. With the build in debug option the code can be tested and validated. The result is a new model or internal that can be used by any user after sharing a dynamic link library (DLL). DLL's enable the encapsulation of proprietary model information while still allowing others to use the model. It also allows the in-house development of only those parts that are really needed while reusing the generic framework of a robust column simulator. This all can be done with minimal programming efforts to keep the focus on describing the performance of the separation device itself. Reactive DistillationThe design and operation issues for reactive distillation processes are more complicated than those of either conventional reactors or conventional distillation columns. The presence of chemical reactions within the column leads to complex interactions between vapor-liquid equilibrium, vapor-liquid mass transfer, intra-catalyst diffusion and chemical kinetics. For such systems the chemical reaction influences the efficiencies to such an extent that the concept loses its meaning. Building a NEQ model of a reactive separation process is not as straightforward as it is for the EQ stage model, in which we simply add a term to account for reaction to the liquid phase material balances. It must be recognized that no single NEQ model can deal with all possible situations; separate models are needed depending on whether the reaction takes place within only the liquid phase or if a solid phase present to catalyze the reaction. We are developing both steady state and dynamic nonequilibrium models of reactive distillation. Our objective is to incorporate these models into Cape-Open ChemSep.
Unsteady state nonequilibrium modelsVarious types of unsteady state models are available where different methods are employed to determine the flows on the trays and packings. Close to flooding the efficiency decreases due to increased mixing on the internals. Only the ChemSep nonequilibrium model with its dispersion flow model can predict how mass transfer of individual species deminishes when column internals flood. This is essential for the proper modeling of upsets in columns where flowrates or concentrations undergo fluctuations. Also, fundamentally sound mass transfer models need to be employed to simulate true column transients, as many "1st generation" models were not formulated with mathematical and physical consistency in mind. Not doing so might for example cause erroneous combinations for the values of mass transfer coefficients and interfacial area. Further Reading
|
|||||
|
|
|||||